- EN
- ES

An official website of the Biotechnology Innovation Organization
Patient safety is central to the research and development of a vaccine. Like all new medicines or treatments, vaccines must pass a rigorous set of tests before being approved by independent, scientific experts at the U.S. Food and Drug Administration (FDA). In fact, the vaccine development process is so difficult that only one out of every five experimental vaccines are successfully approved by the FDA.
First safety steps
Vaccine developers who plan to perform human clinical trials are first required to submit an application to the FDA that includes key information, such as how the potential vaccine is manufactured and any safety and efficacy data already gathered on the potential vaccine.
Along with the application, vaccine developers must recommend a clinical trial protocol that explains what will be tested, how it will be tested, and how success will be measured. Vaccine developers also propose a Data Safety Monitoring Board (DSMB), an independent group of chemists, microbiologists, medical officers, and other experts who oversee the study. The FDA evaluates the proposed protocol and safety board before any clinical work can begin.
Clinical trials
Once the FDA green-lights the application, vaccine candidates undergo three phases of clinical trials. While vaccine developers organize and monitor trials, physicians working in offices and hospitals across the nation and around the world are the ones performing the trials.
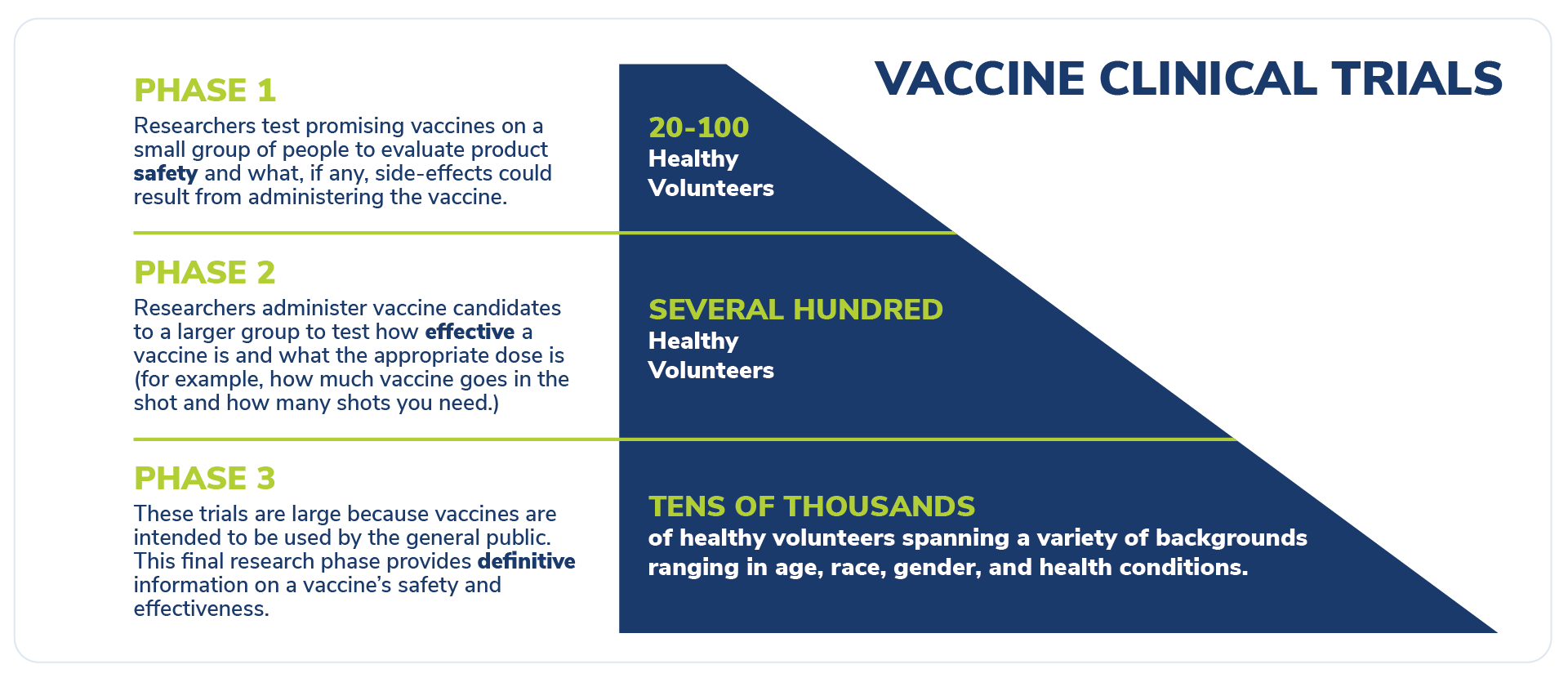
The clinical trial process for vaccines is broken up into three phases, with each phase more and more volunteers are added. By the time a vaccine comes up from approval tens of thousands have participated in the clinical trials.
The gold standard for vaccine development
During Phase 3, or the final phase of testing, researchers determine how effective a vaccine is by looking at those in the clinical trial who contracted the disease and comparing whether they received the vaccine or the placebo. This is what’s known as “event based” or “case based” efficacy, and here’s how that might look in a routine vaccine trial:
In a routine vaccine trial, researchers determine how effective a vaccine is by looking at those in the clinical trial who contracted the disease and comparing whether they received the vaccine or the placebo.
FDA review and long-term safety monitoring
Once data from Phase 3 is compiled, vaccine developers send all safety and efficacy data to the FDA for review. At the same time, the proposed manufacturing site must pass a pre-approval inspection visit where officials ensure the vaccine can be produced safely.
With the clinical trial and manufacturing data in hand, experts at the FDA determine whether the data show the vaccine is safe, effective, and can be manufactured at a high quality and on a consistent basis. Only after these criteria are met, the FDA will provide its stamp of approval for the vaccine.
But the journey doesn’t end there. The FDA, Centers for Disease Control and Prevention (CDC), and vaccine developers continue monitoring the vaccine’s safety and efficacy as long as the vaccine is used. Moreover, your local health care provider serves as yet another line of protection in an already heavily guarded process. They are legally obligated to report any adverse or negative outcomes to the FDA and CDC.
The vaccine development process is designed to ensure any vaccines used by the public have undergone rigorous testing to prove they are safe and effective. Ultimately, the vaccine development process helps make sure we are best equipped to protect our families and communities.
Click here for more information about clinical trials.