- EN
- ES

An official website of the Biotechnology Innovation Organization

Researchers are moving fast to develop vaccines to combat Covid-19. In the past, it would take years to bring a new vaccine through all the regulatory hurdles established by the Food and Drug Administration (FDA) and to approval.
Yet today, less than one year after the first case of Covid-19 was diagnosed in the United States, there are roughly 200 potential vaccines in the works, a handful of which are in the final stages of clinical development. How is this possible?
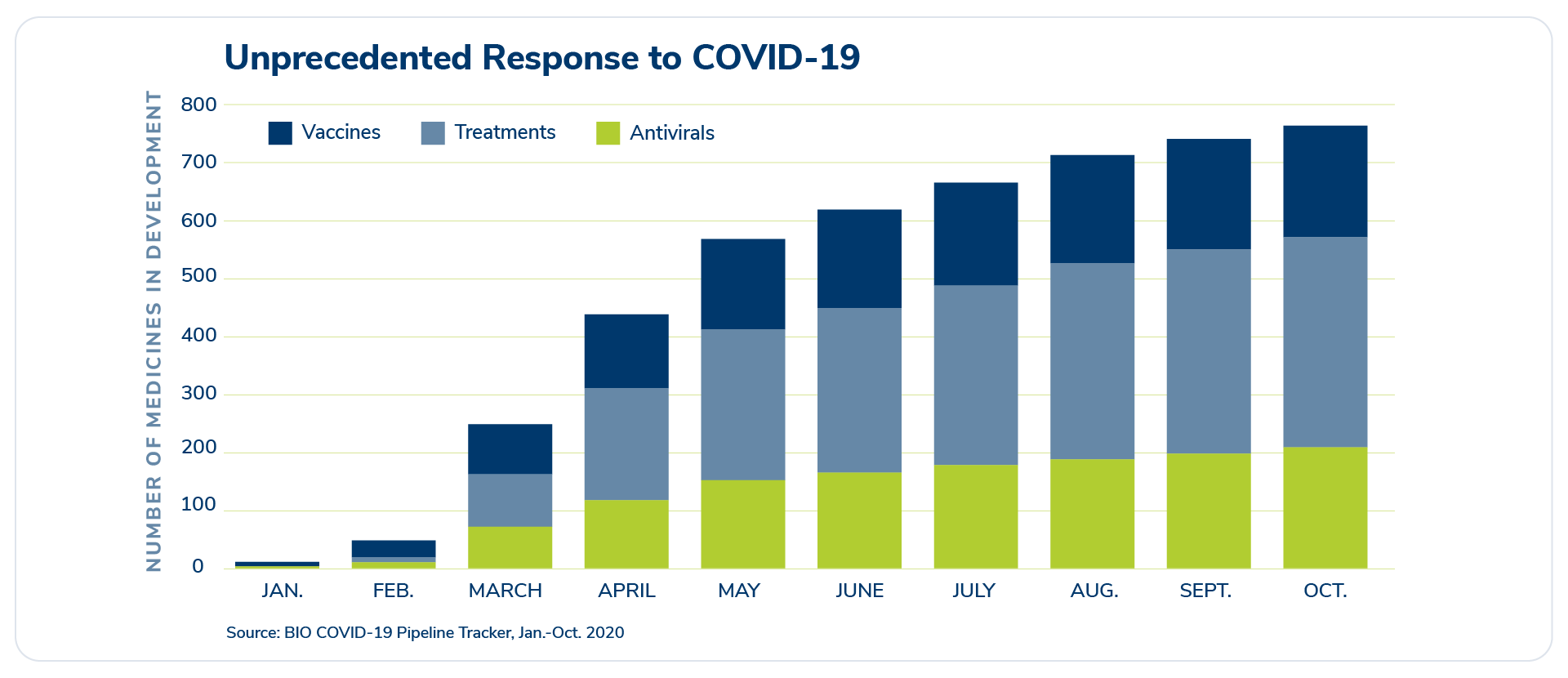
Researchers are moving fast to develop vaccines and treatments to combat Covid-19. The number of medicines in development for Covid-19 continues to increase by month.
Below we explain three important factors that have allowed biopharma researchers to move quickly in the race to find a Covid-19 vaccine:
Novel technologies
Over the past few decades, there have been major scientific improvements that have shortened the time needed for basic research. This includes new technology that allowed researchers to map the genetic code of the coronavirus in just a few short months.
Additionally, scientists didn’t start “from scratch” in their search for a Covid-19 vaccine. While Covid-19 is a novel coronavirus, coronaviruses themselves are not new diseases. In searching for vaccines for other coronaviruses like MERS and SARS, vaccine developers have spent years researching the basic microbiology and chemistry for these different viruses.
With this knowledge, researchers have been able to identify promising vaccine candidates faster than ever before. Innovation in different platforms or approaches for vaccine development, such as viral vector, mRNA and DNA vaccines, and protein expression systems, have been tested and improved over many years to allow us to move more quickly today.
Overwhelmed? These are complex terms that most people have never heard before. Fortunately, the New York Times has a helpful guide for the many different scientific approaches researchers are taking to find a Covid-19 vaccine, including those mentioned above.
The big takeaway: All of these different platforms allow researchers to more quickly attack viruses as they emerge, thereby streamlining the research and development process and ensure we have multiple solutions to address the diverse needs of the global population.
Or, put differently, it’s like a homebuilder planning to build a new house and the foundation has already been set – saving critical time in the construction process.
Collaboration
Over the last several months, there has been incredible collaboration between the public and private sectors with one common goal: end the Covid-19 pandemic quickly and safely. This unprecedented global cooperation has helped accelerate the research and development process.
As we explain in “How are vaccines developed,” the clinical trial protocol outlines important research decisions, such as how the vaccine will be tested and what success looks like. With nearly 200 experimental Covid-19 vaccines in the pipeline, the FDA and National Institutes of Health (NIH) developed a base criteria for all vaccine makers to use. The guidance has made it more efficient for the FDA to compare data and results for different vaccines and has allowed Covid-19 vaccine developers to work from the same playbook to achieve the same goal of slowing the spread of Covid-19. It’s the game plan each team in the race needs to follow.
Example B: Clinical Trial Site Infrastructure
Clinical trials require thousands of participants across different racial, health, and age backgrounds. Without a large and diverse group, it’s difficult for researchers to determine whether a vaccine is safe and effective for people in every family and community.
Finding the right number of participants is always challenging – especially when there are more than 200 vaccines concurrently in the pipeline for a single disease. To save time, biopharmaceutical research companies and the NIH leveraged an existing network of clinical trial sites and a central recruitment website to fast-track the enrollment of clinical trial volunteers and the start of clinical research.
Example C: Manufacturing
Testing the safety and efficacy of a vaccine is just one piece of the puzzle. To bring relief from the pandemic, we also need to produce a supply of vaccines safely, efficiently, and consistently. Biopharmaceutical research companies, government agencies, and non-government partners are actively working together – and have been for months – to scale up the manufacturing process to start producing vaccines as clinical trials are happening. This will allow doses to start being delivered immediately after a vaccine is authorized for use. Ultimately, these efforts will help ensure a more efficient vaccine distribution process to swiftly deliver doses to the people and communities who need them.
Prioritizing resources
Typically, biopharmaceutical research companies work on multiple products at once to develop better treatments for a range of illnesses – from cancer to Alzheimer’s, diabetes to depression, and more.
Since the start of the pandemic, however, researchers have shifted significant resources and time towards finding a safe and effective vaccine for Covid-19. Even though this may impact other important research, it is one more reason why those developing a vaccine have moved so quickly.
Governments around the world and here in the United States have also shifted resources to fighting Covid-19. The FDA, for example, has made some strategic decisions to prioritize its time, oversight, and paperwork related to Covid-19 vaccines. The agency has instituted a “rolling review” process to let the agency’s independent experts analyze research data in real time. This means everyone is following the same regulatory steps necessary for drug development, but with dedicated resources to move more swiftly.
All of these factors – collaboration, innovation, and prioritization – will hasten the development, approval, and distribution of a Covid-19 vaccine without jeopardizing critical safety and efficacy safeguards.