- EN
- ES

An official website of the Biotechnology Innovation Organization
Public health officials and scientific experts are responsible for deciding who should receive a vaccine and when they should receive it. The biopharmaceutical research companies developing vaccines for Covid-19 have no role in the review and recommendation processes.
The Advisory Committee on Immunization Practices
One entity in particular, the Advisory Committee on Immunization Practices (ACIP), helps lead the nation’s efforts to decide how vaccines should be used across the U.S. population. A federal advisory committee to the Centers for Disease Control and Prevention (CDC), this 15-member committee is comprised of independent physicians, immunologists, experts in vaccine development, consumer representatives, and professionals across other medical and public health fields. In addition, the committee includes representatives from other key government agencies and a wide range of outside organizations concerned with vaccinations and public health.
The committee’s primary job is to review all safety, efficacy, and economic data for vaccines and then recommend who should receive the vaccine and when. This process is the same, whether we are discussing a pandemic vaccine or a routine vaccine, like the annual flu shot.

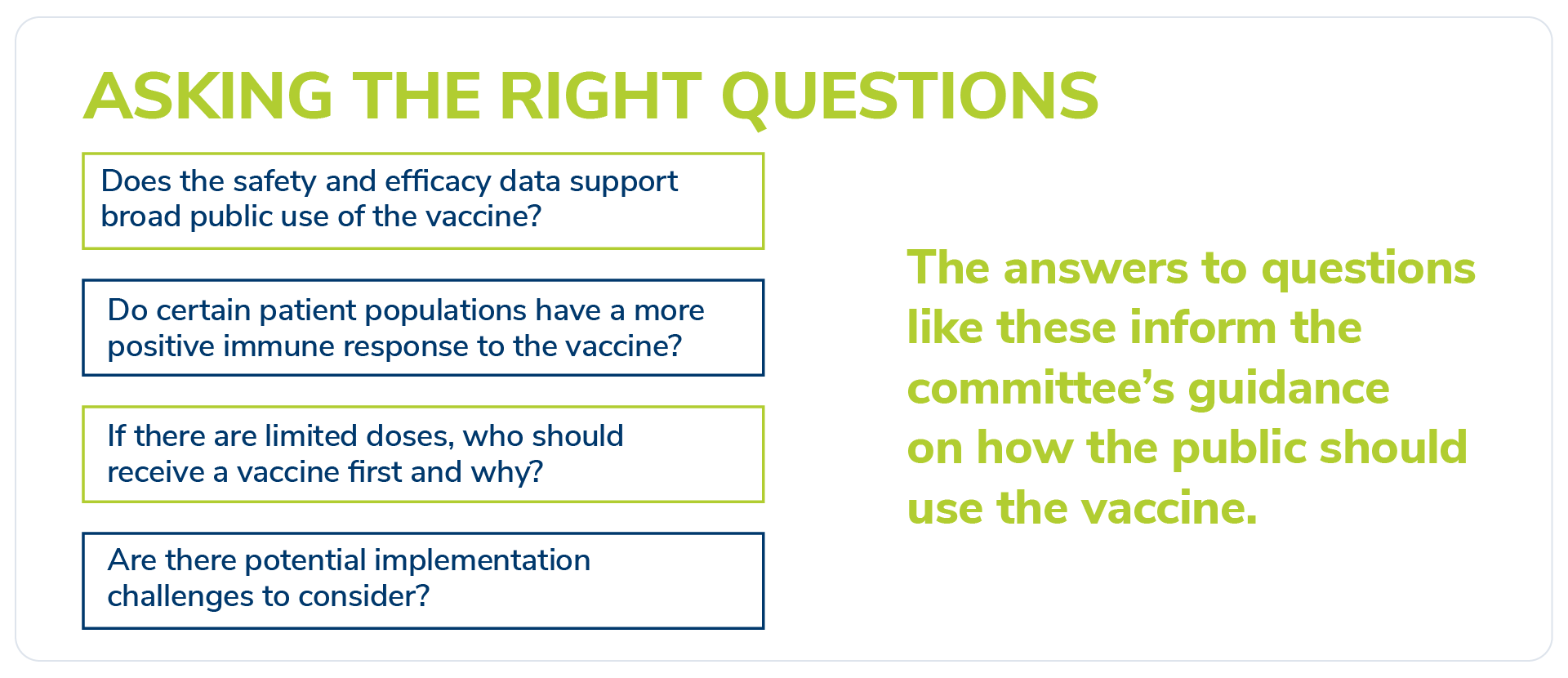
Asking the right questions
Committee members review presentations on the scientific and clinical data for the vaccines at the same time as the Food and Drug Administration (FDA) conducts its intensive regulatory review. Once the FDA approves the use of a vaccine, the committee analyzes all vaccine clinical trial data from that approval. They determine – through a collaborative process – the best use for a vaccine by considering important questions.
Outside collaboration
The answers to these questions and others inform the committee’s eventual guidance on how the public should use a vaccine – or, put simply, who receives a given vaccine and when. That is why it is critical to have a diverse set of voices participating in the discussions. It is also why the ACIP includes members with diverse public health backgrounds, works with key governmental organizations concerned with vaccine access and safety, and collaborates with outside organizations, such as the National Academies. These varying perspectives help the ACIP devise a comprehensive vaccine strategy – and ensure public transparency throughout the process.
Covid-19 vaccine “matching”
In the fight against Covid-19, meeting the different needs of various patient populations will likely require multiple vaccines (a topic we discuss a bit more here). We also need to recognize that once the first Covid-19 vaccine is approved there will be a limited supply for some time. Fortunately, there are dozens of vaccines in development, providing many shots on goal to help win this fight.
The ACIP will closely review all the vaccine clinical trial data submitted to the FDA, including data for different populations based on race, age, and health status. It will also look at current virus trends across the country to determine which populations are most at risk of contracting the disease.
The end goal? To strategically distribute the limited supply of vaccines as efficiently and quickly as possible and to ensure they reach the individuals, families, and communities who need them most.
Learning from recent experience
The H1N1 pandemic that hit the United States and other parts of the world in 2009 offers a lesson for how the ACIP has handled vaccine allocation in past pandemics. As will likely be the case with Covid-19, doses were limited when the vaccine to combat H1N1 first became available. The committee analyzed scientific data – such as who was getting infected with the disease and who was at the highest risk – and then recommended which populations should have first access to the vaccines. They also recommended a tiered vaccine rollout plan for when more vaccines became available, prioritizing the most vulnerable Americans at the top.
You can learn more about the experience with H1N1 here.
The bottom line
Determining who gets a vaccine – and when – is a complex game of chess with lots of moving pieces. The position of each piece impacts all the other pieces on the board. However, each move is backed by the best data and science available to ensure the final outcome is a win for families and communities across the country.