- EN
- ES

An official website of the Biotechnology Innovation Organization
The simple answer is, yes. All vaccines – even those developed in response to a pandemic like Covid-19 – must follow the Food and Drug Administration’s (FDA) strict scientific and regulatory requirements (we explain in more detail what the process looks like here). This means that, even with Covid-19:
- Vaccines are studied using what is globally recognized as the “gold standard” for clinical trials.
- The FDA weighs in on the study design, the protocols a vaccine maker follows during the research process, and the convening of an independent board to monitor safety.
- The number of individuals enrolled in clinical trials must be comparable or greater than the number normally enrolled in vaccine trials.
- Research data is closely scrutinized by independent FDA experts for safety and efficacy.
- The FDA evaluates the manufacturing process for vaccines to ensure consistency, quality, and safety.
- Vaccine developers – working with government agencies and local health care providers – will continue to study and monitor a Covid-19 vaccine years after it receives approval. Learn more about that process here.
Put simply: The development process is the same for Covid-19 vaccines as it is for all vaccines, such as those developed to fight shingles, H1N1 influenza (flu), and other infectious diseases.
Developing a vaccine in a pandemic
Because we are in a public health emergency, critical research, development, and testing for Covid-19 vaccines are happening on a much larger scale than for other infectious diseases.
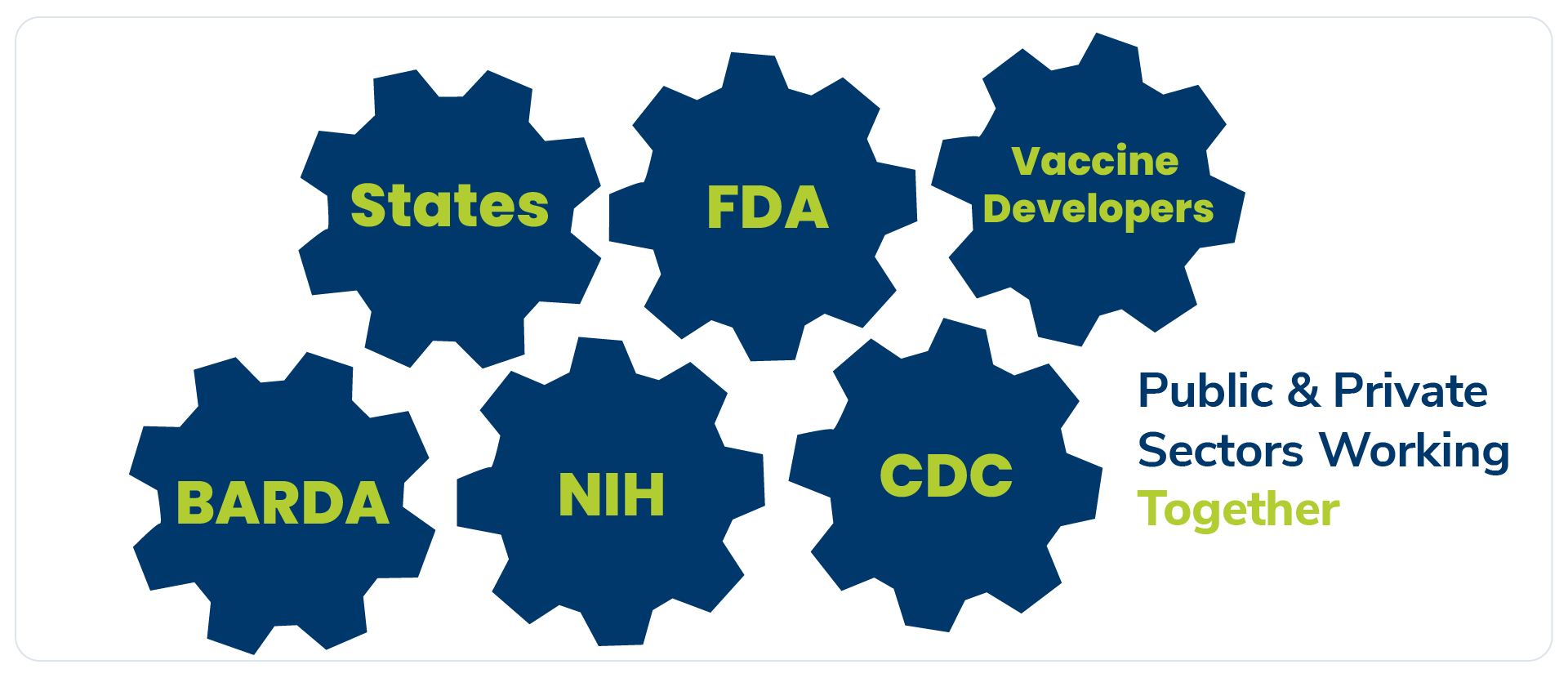
Biopharmaceutical research companies are collaborating closely with key government agencies – including the FDA, National Institutes of Health (NIH), and Biomedical Advanced Research and Development Authority (BARDA) – in an unprecedented effort to develop a vaccine. Vaccine makers are also coordinating with the Centers for Disease Control and Prevention (CDC) and states to plan ahead for distribution. These key partners are working around the clock to combat this pandemic.
In some ways, the effort today is a lot like how the United States landed the first man on the moon. Both public and private partners committed significant time and resources and were racing against the clock to achieve a common mission, and importantly, everyone had to follow the science to accomplish the mission safely.

More scrutiny and transparency
The speed and scale of the Covid-19 response doesn’t change the scientific process or the safety and efficacy standards required to develop a vaccine. Quite the opposite. The added resources and urgency for a vaccine demand even greater scrutiny of the process for Covid-19. This means researchers and public health officials have to be even more open and transparent about their work.
More scrutiny. More transparency. The same gold standard for vaccine development. These are a few reasons why we should all have confidence in the unprecedented response taking place to develop vaccines for Covid-19.