- EN
- ES

An official website of the Biotechnology Innovation Organization
Not at all. Public safety has always been a top priority for the Food and Drug Administration (FDA) and vaccine developers, and that is especially true today in the middle of a pandemic. The vaccine development process is designed with built-in review by multiple independent and expert bodies to ensure all vaccines that reach the public – even vaccines developed during a pandemic – are safe and effective.
You can learn more about the safety systems at work in every step of the clinical trial, approval, and post-approval phases here.
FDA’s historical precedent
For more than a century, the FDA has been the nation’s top watchdog to ensure medicines and vaccines are safe. The organization has a proven track-record of advancing patient interests when it comes to medical innovation. Along with safety, science and transparency are at the heart of everything the FDA does. This includes the multi-layered clinical trial process and the rigorous regulatory approval process for new vaccines. The independent experts who make up these different safety tiers rely on data and science – not politics – to make important decisions. The different checks and balances ensure every medical product that reaches the public has met all regulatory requirements and been thoroughly vetted for safety and efficacy by not just one, but several independent expert advisory groups.

A firm commitment
America’s biopharmaceutical research companies are committed to safety and science. Many industry leaders have publicly promised to move as fast as the data and science allow, but no faster. This promise, which was explicitly outlined in both an open letter and pledge earlier this year, demonstrates a culture of transparency and loyalty to science and safety above all else.
The public has seen this promise in action on more than one occasion.
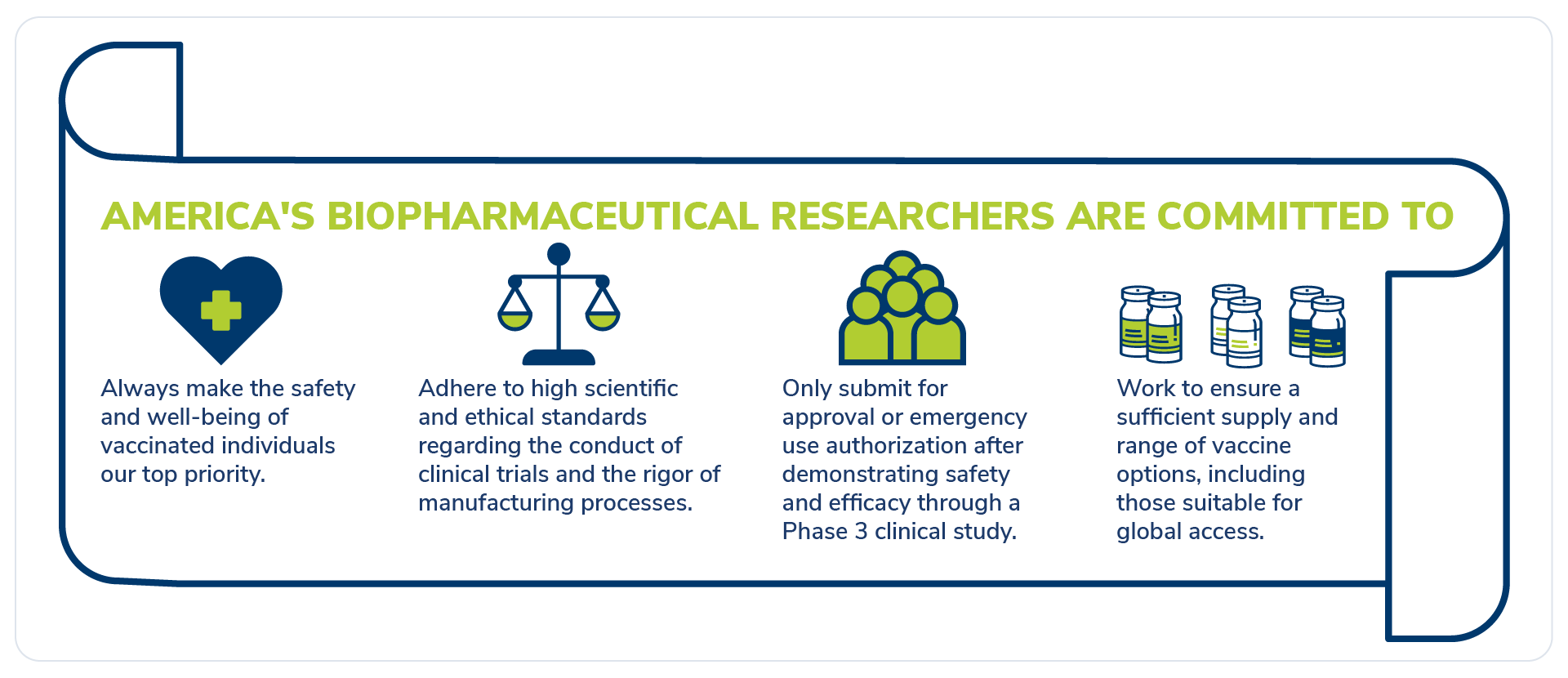
One leader at a biopharma company stated explicitly in an open letter that data alone will dictate the timeline to develop a vaccine. Other researchers racing to develop a Covid-19 vaccine have paused testing to examine the data and ensure clinical trials are operating safely and ethically. Also, as we explain here, the release of FDA guidelines for Covid-19 vaccine development means the public is able to know exactly what researchers are doing. Everyone is taking the time to get this right.
That Covid-19 vaccine developers are adhering to normal clinical trial guidelines, and experiencing normal clinical trial hurdles, is further assurance that the development process for Covid-19 vaccines is working exactly as intended with proper safeguards in place.
Scientists are moving quickly to develop a Covid-19 vaccine, as we explain here. But there is no political agenda fueling their efforts – just science and the common goal of protecting families and communities from this devastating pandemic.